HBB Segcheck v1.2
Cat. No: KBC-110201Beta-thalassemia diagnostic aid kit along with initial screening for common numerical chromosomal abnormalities
Prenatal diagnosis is prone to misdiagnosis for various reasons. Every year, there are numerous legal cases in this field, and medical genetic laboratories spend a significant amount of money and effort to take all necessary precautionary measures to prevent misdiagnosis. Most of these incorrect diagnoses could stem from contamination of the fetal sample with the maternal sample, sample displacement during various diagnostic stages, sample mixing, disomy, uniparental duplication or ADO, crossover, new mutations, and paternity denial, among others.
Kawsar Biotechnology Company has devised an innovative method based on multiple QF-PCR to overcome most causes of misdiagnosis. Each of the ™SegCheck kits contains STR markers linked to a specific gene and additionally has STR markers specific for QF-PCR used for diagnosing aneuploidy of chromosomes 13, 18, 21, X, and Y. The ™SegCheck kits have been designed and developed for diagnosing various genetic diseases. Therefore, multiple markers for the target gene increase the accuracy of the correct diagnosis and minimize the chance of misdiagnosis and prevent incorrect diagnoses.
- Features and benefits include:
- Tracing the disease gene in the family tree
- Resolving the doubt of embryonic sample contamination with the maternal sample
- Resolving the doubt of sample contamination with another sample
- Confirming the authenticity of the embryonic sample and diagnosing the sample displacement error
- Determining the gender of the sample
- Identifying the sample
- Initial screening for chromosomal aneuploidy
- Diagnosing and distinguishing embryonic samples in multiples
- Diagnosing uniparental disomy (either heterodisomy or isodisomy)
- Diagnosing the crossing-over phenomenon in HLA studies and disease occurrence
- Diagnosing embryonic mosaicism
- Gonadal mosaicism (mainly in X-linked disorders)
- Confirming mutation diagnosis results using direct methods such as DNA Sequencing, ARMS, MLPA, Gap PCR, and NGS
The importance of using the ™SegCheck kits lies in the fact that, in most countries, prenatal testing for chromosomal aneuploidies is carried out regularly. Most conventional methods (i.e., first and second-trimester ultrasounds and biochemical screening) have flaws as there are false positives or negatives in diagnosing chromosomal aneuploidies. QF-PCR has long become a selective method for quickly diagnosing common chromosomal aneuploidies. However, techniques such as QF-PCR and karyotyping should be requested by specialists and performed on selected embryonic samples. Aware of these deficiencies, Kawsar Biotechnology has produced the ™SegCheck kits designed for over 60 diseases, allowing laboratories to identify any disease along with initial screening for aneuploidies using the QF-PCR method without requiring sampling and additional costs. Even NIPT (non-invasive prenatal testing) only tests the embryo sample for aneuploidy. In contrast, the ™SegCheck kit can examine both the disease gene and aneuploidies for every embryo sample undergoing prenatal diagnostic testing. Each ™SegCheck kit includes specific STR markers from chromosomes 13, 18, 21, and the sex chromosomes along with specific disease STR markers.
The STR markers are chosen from 4-nucleotide repeats for less complex analysis and easy interpretation. The alleles resulting from these STR markers can help in verifying the sample’s identity, dismissing maternal cell contamination, and many other benefits in one test. Thus, these chromosomal markers can also be used to examine the presence of aneuploidy for the tested chromosomes. If aneuploidy is suspected, the sample can be analyzed with more markers using the QF-PCR method, such as with the AneuQuick™ Kit v3.2 or AneuQuick™ Extra Plus Kit v1.2.
Every genetic diagnostic laboratory faces issues such as sample mixing and maternal cell contamination when conducting prenatal diagnosis. These errors can lead to incorrect diagnoses and false-negative screenings. The ™SegCheck can aid any medical genetic laboratory conducting prenatal diagnosis. Since these kits contain markers within or adjacent to a specific disease gene and segregate, these markers can be used for tracking segregation patterns and linkage analysis. Drawing a haplotype to confirm the inheritance of a gene mutation in an embryo is essential. For instance, suppose both parents have a similar mutation. In that case, allelic drop out during direct mutation diagnosis in the embryo might indicate homozygosity, whereas haplotype drawing and analysis in a family show that it is indeed heterozygous because of ADO, where the other gene is not amplified, thus carrying the risk of miscarriage. The reason ™SegCheck kits can detect ADO is that each kit contains between 4 to 8 STR markers, and a sample cannot have ADO for all these markers.
This approach utilized in these kits will be valuable for most molecular medical genetic laboratories conducting prenatal diagnosis. However, they can also be used by other researchers for various purposes such as genetic or hereditary segregation or even mosaicism.
Hemoglobinopathies (all types of genetic diseases caused by defects in the hemoglobin gene, including beta-thalassemia, sickle-cell anemia, and hemoglobin H disease) are the most common single-gene disorders worldwide. Beta-thalassemia is prevalent in the Middle East and Mediterranean regions, while sickle-cell anemia is more common in Africa and among people of African descent, and alpha-thalassemia disorders like hemoglobin H disease are more prevalent in East and Southeast Asia. The gene responsible for beta-thalassemia and sickle-cell anemia is called beta-globin. The chromosomal location of this gene is 11p15.4. Most prenatal diagnoses conducted worldwide are related to hemoglobinopathies.
The HBB SegCheck™ Kit v1.2 is a molecular diagnostic kit that identifies carriers or those affected by beta-thalassemia and sickle-cell disease within a short period. This kit primarily uses STR markers and the QF-PCR technique to analyze segregation and genetic linkage haplotyping, thus diagnosing embryo carrier status or disease presence. Utilizing STR markers, along with direct mutation detection, makes prenatal diagnosis more accurate and reliable. Along with detecting hemoglobin gene defects, the HBB SegCheck™ v1.2 kit includes autosomal STR markers for diagnosing aneuploidy of chromosomes 21, 18, 13, X, and Y using the QF-PCR method.
The diagnostic HBB SegCheck™ v1.2 kit is based on 14 markers, including 5 high-heterozygosity STR markers upstream and downstream of the HBB gene and 9 markers for primary screening of common chromosomal disorders (3 markers for chromosome 21, 2 markers for chromosome 18, and 2 markers for chromosome 13) and gender determination.
This kit is applicable to DNA samples purified from blood, amniotic fluid (AF), fetal chorionic villus sampling (CVS), and DBC cards (Kawsar Biotechnology’s DNA storage card), Blood Lysis Buffer (BLB), Amniotic Cell Lysis Buffer (AFL), and Chorionic Villi Lysis Buffer (CVL).
For more information on the product, how to use it, and result analysis, please refer to the manual.
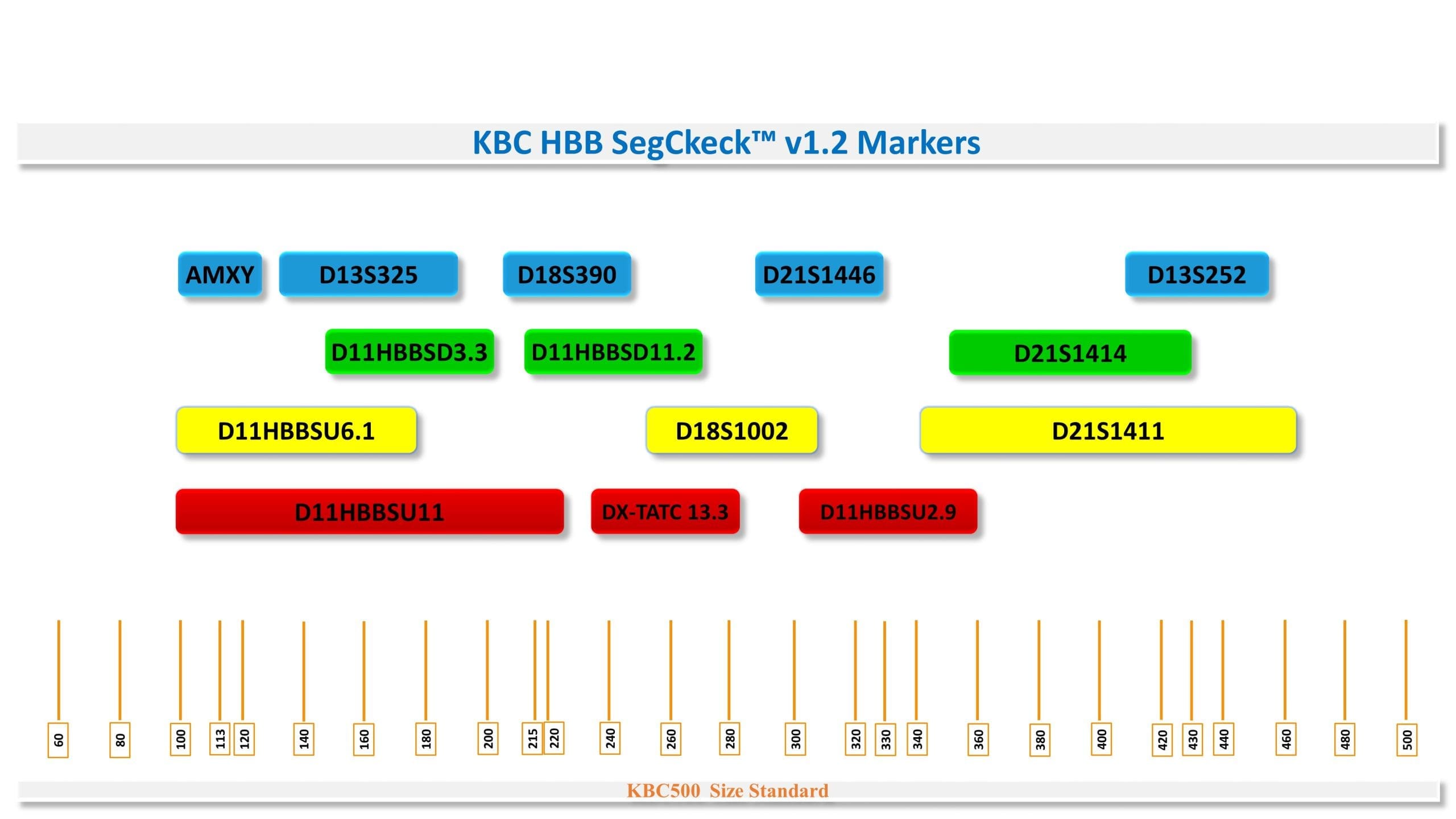
D11HBBSU2.9،D11HBBSU6.1 ،D11HBBSU11 ،D11HBBSD3.3 ،D11HBBSD11.2 ،D21S1446 ،D21S1414 ،D21S1411 ، D18S390،D18S1002 ، D13S325، D13S252، DX-TATC 13.3 و AMX.
موقعیت مارکرهای STR کیت HBB SegCheck™ v1.2 نسبت به ژن HBB:

This kit is compatible with 5-color capillary electrophoresis systems such as the ABI PRISM 3130/3130xl/3500/3500xL Genetic Analyzer with 30, 50, or 80 cm capillary sizes.
User Manual
Quick Protocol
MSDS
Analysis Assistant
Profile





















Reviews
There are no reviews yet.