AneuQuick™ v4.2
Cat. No: KBC-110111Prenatal molecular diagnostic kit for common chromosomal numerical abnormalities (aneuploidy).
The AneuQuick™ Kit v4.2 is a quantitative fluorescence polymerase chain reaction (QF-PCR) molecular diagnostic kit used for prenatal screening and rapid detection of common chromosomal numerical abnormalities.
When the number of chromosomes is not a multiple of 23 (for example, instead of 46 chromosomes, a person has 47 chromosomes), this condition is referred to as chromosomal numerical abnormalities or aneuploidy. The most common aneuploidies are chromosomes 21, and the sex chromosomes X and Y. Other detectable aneuploidies with this kit include trisomies of chromosomes 13 and 18. Chromosomal abnormalities can also be investigated through cytogenetic methods and performing karyotyping, which typically requires at least two weeks for fetal cells to grow and the potential aneuploidy status to be assessed. However, recently, the new molecular method QF-PCR has become significantly prevalent as it can be performed in a shorter time and offers cost-effectiveness and very high accuracy. This method enables precise results within a maximum of 5 hours.
The AneuQuick™ v3.2 kit contains 26 STR markers from chromosomes 13, 18, 21, X, and Y (21 STR markers with high heterozygosity and 4 markers for gender determination and sex chromosome abnormalities). By adding the marker 11X to the KBC AneuQuick™ v3.2 kit, it is released as AneuQuick™ v4.2. The 7X and 11X marker are also segmental duplication markers included in the kit to distinguish X chromosome monosomy from homozygosity and to detect Turner syndrome. These STR markers in both versions are simultaneously examined using quantitative fluorescence polymerase chain reaction (QF-PCR). Therefore, the AneuQuick™ v3.2 & v4.2 kits can be used in the following cases:
- Diagnosis of Down syndrome (Trisomy 21)
- Diagnosis of Edwards syndrome (Trisomy 18)
- Diagnosis of Patau syndrome (Trisomy 13)
- Diagnosis of Turner syndrome (X, 45)
- Diagnosis of Klinefelter syndrome (XXY, 47)
- Diagnosis of Jacob syndrome (XYY, 47)
- Diagnosis of Triple X syndrome (XXX, 47)
- Other aneuploidies related to chromosomes X and Y
- Diagnosis of triploidy and tetraploidy
- Gender determination
It is worth mentioning that QF-PCR is a diagnostic method, while NIPT is a screening method. Therefore, the AneuQuick™ v4.2 kit is a suitable alternative for confirming the test after the NIPT results have been positive.
Features and Benefits
- Analysis of 27 markers in a single reaction
- Use of STR markers with high heterozygosity
- Rapid diagnostic follow-up for NIPT or first and second trimester screening tests
- Optimized for working with various human DNA sources
- Easy to use
This kit can be used on DNA samples extracted from blood, amniotic fluid (AF), chorionic villus sampling (CVS), and DBC cards (DNA storage cards by KBC). It is compatible with blood lysis buffer (BLB), amniotic fluid lysis buffer (AFL), and chorionic villus lysis buffer (CVL).
For further information on the product, its usage, and result analysis, please refer to the user manual.
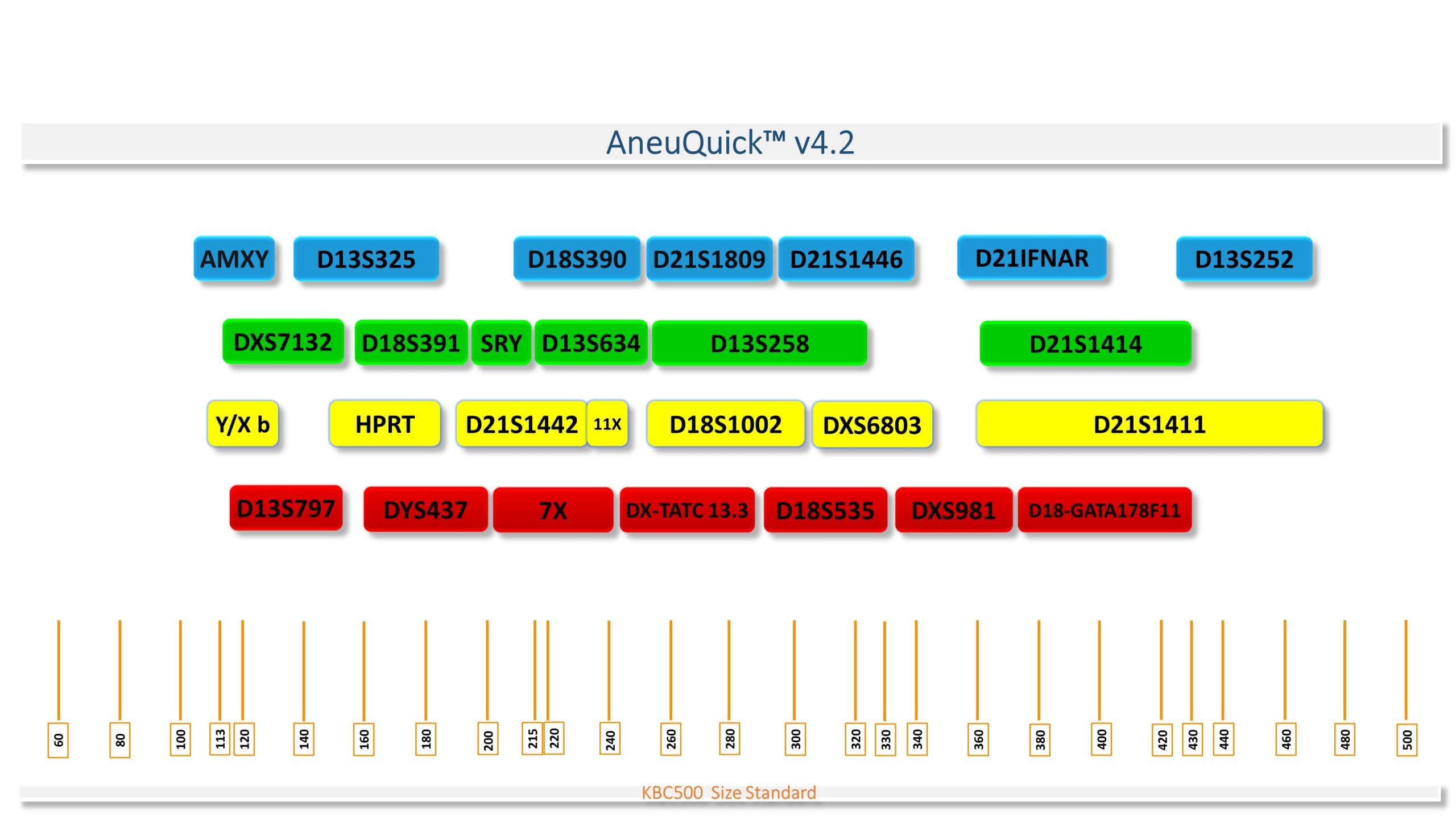
Figure 1. Map of AneuQuick v3.2 kit marker
There are 27 markers in the AneuQuick Kit v4.2, 28 in the AneuQuick™ Plus Kit v1.2, and 32 in the AneuQuick™ Extra Kit v1.2.
The markers in these kits, amplified through multiplex fluorescent-based PCR, provide higher detection capability to medical genetic specialists. The inclusion of creative SMA detection (by adding exon 7 of the SMN1 and SMN2 genes in the AneuQuick™ Plus Kit v1.2) is a notable advantage for any laboratory. It enables simultaneous detection of cases where the fetal sample appears normal in terms of aneuploidy but is affected by a severe and costly disease like SMA.
Table 1. Comparison Table
| ™AneuQuick
Kit 3.2 |
™ AneuQuick
Kit 4.2 |
AneuQuick™ Plus
Kit v1.2 |
AneuQuick™ Extra
Kit v1.2 |
Features |
| 26 | 27 | 28 | 32 | Total markers |
| 2 | 2 | 2 | 2 | Y markers |
| 6 | 6 | 6 | 5 | X marker |
| 2 | 2 | 2 | 2 | X/Y markers |
| 6 | 6 | 6 | 8 | Chr. 21 marker |
| 5 | 5 | 5 | 8 | Chr. 18 marker |
| 4 | 4 | 5 | 6 | Chr. 13 marker |
| 1 | 2 | 1 | 1 | Segmental
Duplication |
| û | û | ü | û | SMA Detection |
| 5 | 5 | 5 | 6 | 5or 6 dye
system |
| ü | ü | ü | ü | Single tube
multiplex |
| 3130/3130xl
3500/3500xL |
3130/3130xl
3500/3500xL |
3130/3130xl
3500/3500xL |
3500/3500xL | ABI GA
Systems |
This kit is compatible with 5-color capillary electrophoresis systems such as ABI PRISM 3130/3130xl/3500/3500xL Genetic Analyzer with capillary sizes of 30, 50, or 80 centimeters.
User Manual
Quick Protocol
Analysis Assisstant
MSDS
Profile





















Reviews
There are no reviews yet.